Vacancies

We are always on the lookout for talented Ph.D. and Postdoctoral candidates. Inquiries are welcome from graduates and postgraduates who are either self-funded or motivated to apply for research funding. Interested students are advised to clearly read the below information regarding different funding and then can contact Amin for support in applications.
Funded Open Positions
None currently available
Funding Opportunities:
Postdocs:
- Marie Sklodowska Individual Fellowships
- Fonds de la Recherche Scientifique – FNRS
- Chargé de recherches (CR)
- IF@ULB
PhD:
Internships:
We host visiting internships (a minimum of 12 continuous weeks) from students with a background and expertise that fit with the projects in the lab.

| BioMatter lab works at the interface of polymer science, physical chemistry, and biology. The overall focus of the lab is the fundamental understanding and development of biohybrid/bioinspired materials for biomedical applications with a specific emphasis on biomaterials engineering and tissue regeneration to address specific problems related to tissue development, repair, and regeneration. Although significant advances in tissue engineering have been made in recent years, the continued lack of organs and tissue for transplantation calls for the development of innovative treatment alternatives. To achieve this, we are working on developing mechanically and structurally dynamic biomaterials, microfabrication, and matrix manipulation techniques to recreate complex cell-matrix interactions and model tissue morphogenesis and disease. We combine engineering, chemistry and biology to design biomaterials that control and direct the interaction with cells. While most of our target applications lie within biomedical engineering e.g., cell encapsulation, biomedical devices, and tissue engineering, we also apply our engineered hydrogels in food, nutraceutical delivery, agricultural, and environmental applications. https://biomatter.ulb.be/ Amin Shavandi (Head of the lab) |
Research theme: Biomass valorization, Sustainability and the circular economy
Optimization of in-situ catalyst-free biodiesel production from insect waste
Biodiesel has gained attention as a potential alternative to fossil fuels due to its renewability and sustainability, with its renewability further enhanced when inedible waste biomass is employed as the feedstock1. Indeed, the production of biodiesel from waste streams such as insect residues offers a promising solution to the growing environmental and food-fuel conundrum that is associated with conventional biodiesel feedstocks such as vegetable oils2. To this regard, an investigation into in-situ catalyst-free biodiesel production from insect residues, under subcritical conditions, will be undertaken. This in-situ biodiesel production pathway constitutes an intensification technology approach that eliminates the need for preliminary lipid recovery and purification from the insect residue by facilitating the conversion of lipids to biodiesel within the biomass matrix. Subcritical conditions refer to temperatures and pressures below the critical point of the selected solvent that is responsible for catalyst-free alkyl ester formation, which constitutes the precursor to the target biodiesel product. The optimization process to be explored will be based on the Box-Behnken experimental design, which will enable the determination of the optimal conditions for the process parameters such as particle size, biomass-solvent ratio, time, and temperature3. The optimally produced biodiesel product will be assessed with respect to the biodiesel ASTM D6751 and EN 14214 quality standards, for density, viscosity, acid value, and cetane number properties and the sufficiency of the biodiesel product discussed1. Finally, in accordance with the circular economy paradigm, the physicochemical properties of the solid residue generated will be assessed and suitable uses proposed.
Keywords: subcritical in situ transesterification; insect biomass residues; catalyst-free; biodiesel; circular economy.
Requirements
- The candidate should have an excellent academic background preferably a BSc (or BEng.) degree in Chemical/Process/Biomaterial Engineering with a minimum of a 2.1 graduating grade point.
- The candidate should be motivated and have a strong desire to learn and improve.

References
1. Okoro, O. V.; Sun, Z.; Birch, J., Meat processing dissolved air flotation sludge as a potential biodiesel feedstock in New Zealand: A predictive analysis of the biodiesel product properties. Journal of Cleaner Production 2017, 168, 1436-1447.
2. Okoro, O. V.; Sun, Z.; Birch, J., Meat processing waste as a potential feedstock for biochemicals and biofuels – A review of possible conversion technologies. Journal of Cleaner Production 2017, 142, 1583-1608.
3. Okoro, O. V.; Nie, L.; Waeytens, J.; Hamidi, M.; Shavandi, A., Thermochemical Liquefaction of Pomace Using Sub/Supercritical Ethanol: an Integrated Experimental and Preliminary Economic Feasibility Study. BioEnergy Research 2022.
Contact: oseweuba.okoro@ulb.be and amin.shavandi@ulb.be
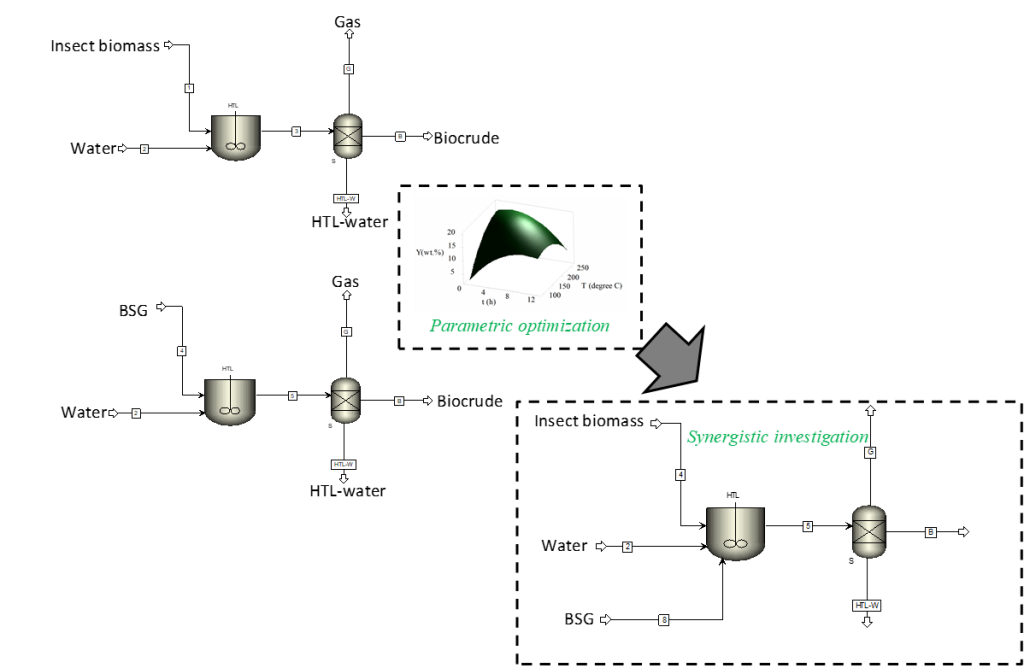
Enhancing Hydrothermal Co-liquefaction of Insect and Brewer’s Spent Grains Biomass: A Synergistic and Parametric Optimization Study
The hydrothermal liquefaction (HTL) technology facilitates the direct conversion of wet biomass into biocrude while circumventing the need for a preliminary drying operation. Biocrude constitutes the main product of the HTL process and can be used as a fuel or a source of biochemicals (1) due to its favorable higher heating value (>30 MJ/kg) (2, 3) and its content of high value compounds (e.g. esters, aromatics, alkanes etc.), respectively. The HTL process exploits water’s ability to alter its thermodynamic properties as its critical condition (Tc = 373.95 °C, Pc = 22.064 MPa)1, 4 is attained, with the polarity of water transitioning from polar to less polar. Due to the potential of utilizing HTL for value extraction from biomass waste, this project will seek to incorporate synergistic effects via co-liquefaction and parametric optimization approaches in an attempt to further enhance the overall process efficiency. In this regard, highly polluting waste streams of insect biomass (ISB) and brewer’s spent grains (BSG) will be assessed as promising HTL feedstocks. The project hypothesizes that by combining ISB and BSG, it may be possible to enhance the overall process efficiency of a low temperature (200 oC <x<300 oC)1 HTL process. During the course of the project, separate parametric optimization studies of ISB and BSG will be undertaken and the optimal conditions for enhanced biocrude production for the different biomasses determined. The compositional assessments of the optimally produced biocrude products will also be undertaken. Different blending mass ratios (1:0, 3:1, 1:1, 1:3, 0:1) of ISB and BSG will then be prepared and subsequently subjected to Hydrothermal Co-Liquefaction (HTcL) while imposing the determined conditions for optimal HTL for ISB and BSG. The resulting synergistic or antagonistic effects of the blending process on biocrude yields will then be determined. In addition to the quantitative effects of blending ISB and BSG on the HTcL process, the effect on the biocrude composition and quality will also be assessed. It is anticipated that this holistic study will inspire innovation in the field of HTcL and further promote the transition toward a more sustainable and resource-efficient Belgium.
Keywords: hydrothermal liquefaction; brewer’s spent grains; insect biomass; optimization; synergizing effects; sustainability
Contact: oseweuba.okoro@ulb.be and amin.shavandi@ulb.be
References
1. Okoro, O. V.; Sun, Z.; Birch, J., Meat processing waste as a potential feedstock for biochemicals and biofuels – A review of possible conversion technologies. Journal of Cleaner Production 2017, 142, 1583-1608.
2. Okoro, O. V.; Sun, Z., The characterisation of biochar and biocrude products of the hydrothermal liquefaction of raw digestate biomass. Biomass Conversion and Biorefinery 2021, 11 (6), 2947-2961.
3. Ellersdorfer, M., Hydrothermal co-liquefaction of chlorella vulgaris with food processing residues, green waste and sewage sludge. Biomass Bioenergy 2020, 142, 105796.
4. Okoro, O. V.; Sun, Z.; Birch, J., Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies. 2018, 8 (11), 2290.
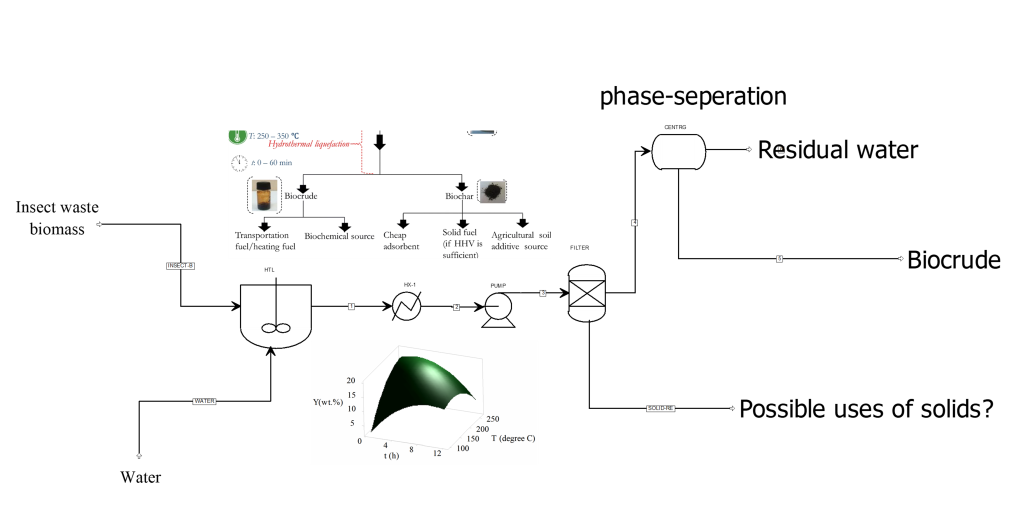
Hydrothermal liquefaction of insect waste residues: an experimental optimization and economic assessment study
Hydrothermal liquefaction (HTL) constitutes a promising technology that facilitates the conversion of wet biomass into biocrude. The biocrude could serve as either a renewable energy source or as a source of biochemicals1. The HTL process is based on water’s ability to change its thermodynamic properties at conditions close to its thermodynamic critical point (Tc = 373.95 °C, Pc = 22.064 MPa)1, 2. During hydrothermal liquefaction, as the critical state of water is reached (i.e., at temperatures 100 -374 °C), its polarity and dielectric constant properties change due to the weakening of hydrogen bonds and an increase in its ionization constant (Kw). However, beyond subcritical conditions, the ionization constant (Kw) decreases as conditions get closer to the critical point (374 °C and 22.1 MPa)1, making the polar nature and the dielectric constant of water to reduce from 80 in ambient conditions to about 20 in the critical region1. Recognizing the potential of this property of water to promote biomass valorization, the project will investigate the optimal HTL conditions for insect waste biomass liquefaction. In the study, the reaction temperature, particle size, reaction time, and biomass-to-water ratio will be investigated as process parameters and the optimally produced biocrude assessed. Further investigations will also be undertaken to assess the preliminary economic performance of the optimally operating HTL process, with discussions comparing the existing unsustainable waste management practices (i.e., use of landfills) and the proposed HTL approach presented. At the end of the project, the optimal HTL conditions for insect biomass liquefaction would have been determined, and the physicochemical characteristics of the biocrude product and comparative economic performance of the proposed technology would also have been assessed. This project will be instructive in ascertaining the feasibility of future explorations of large-scale Insect-biomass HTL plants in Belgium, for the promotion of the circular economy and for the benefit of all Belgians.
Keywords: hydrothermal liquefaction; insect biomass residues; optimization; circular economy.
Requirements
- The candidate should have an excellent academic background preferably a BSc (or BEng.) degree Chemical/Process/Biomaterial Engineering with a minimum of a 2.1 graduating grade point.
- The candidate should be motivated and have a strong desire to learn and improve.
References
1. Okoro, O. V.; Sun, Z.; Birch, J., Meat processing waste as a potential feedstock for biochemicals and biofuels – A review of possible conversion technologies. Journal of Cleaner Production 2017, 142, 1583-1608.
2. Okoro, O. V.; Sun, Z.; Birch, J., Prognostic Assessment of the Viability of Hydrothermal Liquefaction as a Post-Resource Recovery Step after Enhanced Biomethane Generation Using Co-Digestion Technologies. 2018, 8 (11), 2290.
Contact: oseweuba.okoro@ulb.be and amin.shavandi@ulb.be
Research theme: Biomaterials and tissue engineering
Hydrogel-based scavengers of ROS and oxygen generators for mitigating inflammation
The development of certain diseases such as atherosclerosis, myocardial infarction, cancer, and chronic inflammation has been linked to high levels of ROS (reactive oxygen species). Therefore, it is crucial to create materials that can reduce the harmful effects of excessive ROS generation locally. However, hypoxia (a condition of low oxygen levels) can worsen inflammation by causing abnormal ROS production. To address this issue, the proposed solution is to develop modified methacrylate silk fibroin hydrogels (SiMA) that contain calcium peroxide-encapsulated fluorinated hyaluronic acid (HA) particles. The objective is to alleviate hypoxia and scavenge ROSs. To achieve this goal, several steps must be taken: A) Conjugate perfluorocarbon groups onto HA, B) Encapsulate calcium peroxide into fluorinated HA particles, C) Modify silk fibroin with methacrylate groups and catalase, D) Incorporate the synthesized particles into modified SiMA gels, E) Evaluate the physical and chemical properties of the developed hydrogels. This research aims to create a hydrogel that can reduce the harmful effects of excessive ROS generation locally and alleviate hypoxia, which could be beneficial in treating diseases linked to high levels of ROS.

Related literature:
- [1] Z. Li, Y. Zhao, H. Huang, C. Zhang, H. Liu, Z. Wang, M. Yi, N. Xie, Y. Shen, X. Ren, A Nanozyme‐Immobilized Hydrogel with Endogenous ROS‐Scavenging and Oxygen Generation Abilities for Significantly Promoting Oxidative Diabetic Wound Healing, Advanced Healthcare Materials 11(22) (2022) 2201524.
- [2] J. Ding, Y. Yao, J. Li, Y. Duan, J.R. Nakkala, X. Feng, W. Cao, Y. Wang, L. Hong, L. Shen, A reactive oxygen species scavenging and O2 generating injectable hydrogel for myocardial infarction treatment in vivo, Small 16(48) (2020) 2005038.
Contact: Pejman.ghaffari.bohlouli@ulb.be, and Amin.shavandi@ulb.be
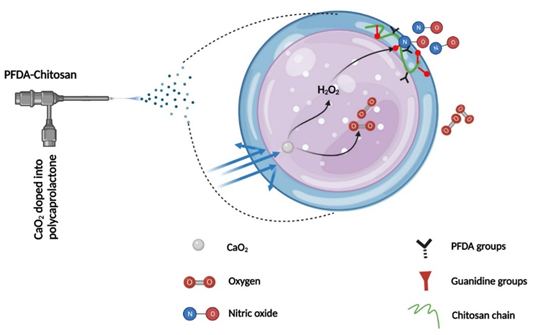
Synergistic oxygen and nitric oxide generation using core-shell particles
Chronic wounds in diabetics can be difficult to treat due to a complex and severe inflammatory microenvironment that includes biofilm formation, excessive reactive oxygen species (ROS), hypoxia, and insufficient nitric oxide (NO) synthesis. To address these challenges, we propose synthesizing core-shell particles that can generate both O2 and NO simultaneously and control their release rate to mitigate hypoxia and prevent infection. We hypothesize that by encapsulating calcium peroxide (CaO2) into polycaprolactone (PCL) particles, we can generate H2O2 and O2 gradually through a reaction with water. The produced H2O2 can then react with guanidine groups on chitosan to generate NO. Perfluorocarbon groups (PFCs) on chitosan will form an NO and O2 buffering shell that can trap excess gas release and release it in a controlled, sustained manner. To achieve this aim, several steps are required: A) Conjugating 40-45% PFC groups on the chitosan chain, B) Replacing the remaining amine groups of chitosan with guanidine groups through a chemical reaction, C) Synthesizing core-shell particles that generate and control the release of O2 and NO, D) Evaluating the kinetics of generated O2, H2O2, and NO from the particles, and E) Evaluating the physical and chemical properties of the developed materials. The proposed research aims to develop a novel approach to address the complex and severe inflammatory microenvironment in chronic wounds in diabetics using core-shell particles that generate both O2 and NO and control their release rate, potentially leading to improved healing outcomes.
Related literature:
[1] C. Tu, H. Lu, T. Zhou, W. Zhang, L. Deng, W. Cao, Z. Yang, Z. Wang, X. Wu, J. Ding, Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties, Biomaterials 286 (2022) 121597.
Contact: Pejman.ghaffari.bohlouli@ulb.be, and Amin.shavandi@ulb.be
Hydrogel vascular grafts reinforced with melt-electrowritten (MEW) polycaprolactone (PCL) lattices
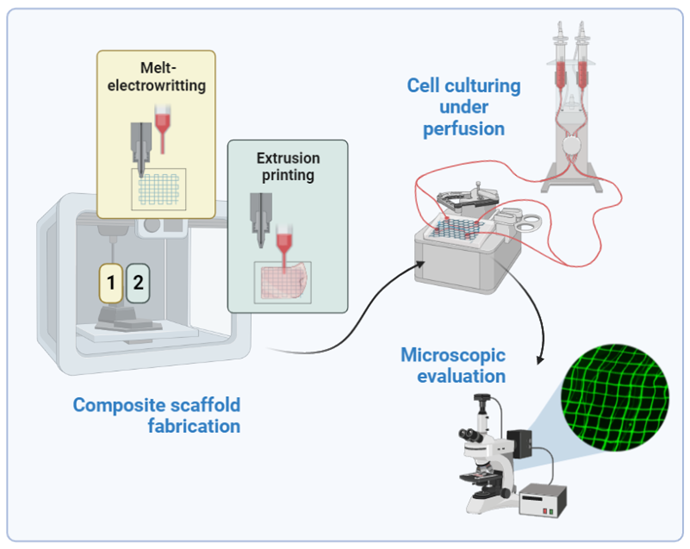
By providing oxygen and nutrients to the cells, as well as eliminating metabolic waste, vascularization is a vital factor in the success of tissue engineering and yet one of its main challenges. Despite multiple advantages, natural polymers used for hydrogel creation have poor mechanical properties limiting their applications. Melt-electrowriting (MEW) offers the possibility to modify the physical characteristics of multi-material constructs comprising fibers and provide a scaffolding to enhance cell survival and ingrowth properties. Such multi-material MEW processes can be adapted to a wide range of mechanical properties, and thus enable target tissue-specific adjustments to obtain ideal scaffold properties. Reinforcing MEW frames embedded in hydrogel can affect the toughness and elastic modulus of the construct, helping to maintain the designed dimensions and architecture.
Keeping that in mind this project combines MEW approach and extrusion printing of a soft hydrogel material to overcome current limitations associated with the creation of artificial vasculature. Additionally, it will investigate whether microstructure fibers can facilitate the specific alignment of cells. The projects’ tasks will cover, the design and fabrication of PCL (polycaprolactone) grids and fibers utilizing melt-electrowritting technique, followed by a process of extrusion printing of soft hydrogel material (gelatin and hyaluronic acid) to provide a cell-friendly interaction site. Composite scaffolds of preferable properties in terms of cell adhesion and durability will be further tested with in-house prepared PDMS chips, by subjecting the scaffolds to a continuous flow condition. Hopefully, the resulting scaffolds can become promising matrices to support vascularization processes addressing the challenge of angiogenesis within hydrogel-based tissue scaffolds.
Related literature:
- https://doi.org/10.1038/s41598-022-24275-6
- https://doi.org/10.3389/fbioe.2020.00793
- https://doi.org/10.1002/adhm.201800418
Contact: julia.siminskastanny@ulb.be and amin.shavandi@ulb.be
Melt-electrowritten (MEW) scaffold decorated with growth factors as a versatile matrix to guide angiogenesis
Lack of a vasculature system within a three-dimensional tissue model can significantly hinder the practical application of such constructs, especially in vivo. Blood vessels are vital to ensure cellular survival and enable tissue restoration first ex vivo and then in vivo. Therefore, the project is guided by a hypothesis that the vascularization of the artificial tissue can be overcome by developing a melt-electrowritten substrate in a form of a lattice decorated with growth factors (GFs) that could help to guide angiogenic processes in matrices composed of different hydrogels for tissue regeneration. Briefly, the projects’ tasks will cover, the design and fabrication of PCL (polycaprolactone) grids with different pore geometries utilizing melt-electrowritting technique, followed by a chemical modification of the polymer (amination) to ease the process of scaffolds decoration with GFs. Subsequently, PCL scaffolds will be tested for their cytocompatibility, stability in simulated body fluid (SBF), and resistance to tensile stress. MEW scaffolds showing the best properties in terms of cell adhesion and durability will be further tested with in-house prepared PDMS chips, by subjecting the scaffolds to a continuous flow condition. Hopefully, the designed PCL scaffolds can become promising matrices for the guidance of angiogenic processes in hydrogel materials helping the creation of artificial tissues with clinically relevant dimensions.
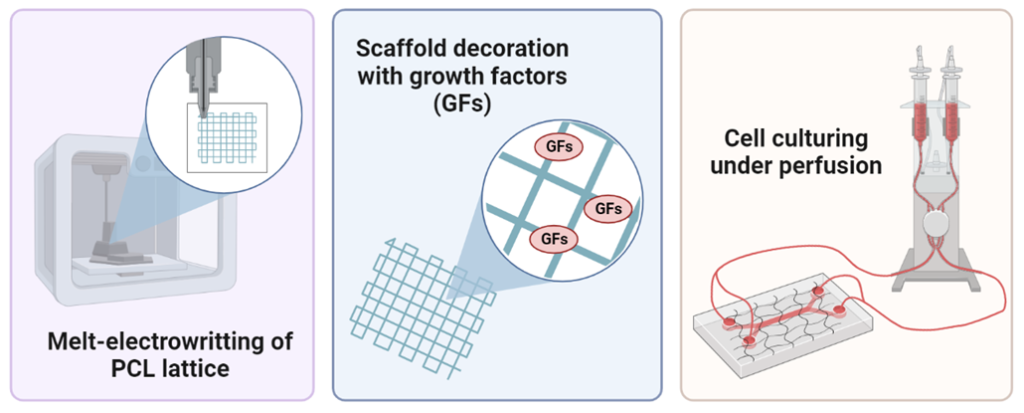
Related literature:
- https://doi.org/10.1038/s41598-022-24275-6
- https://doi.org/10.3389/fbioe.2020.00793
- https://doi.org/10.1002/adhm.201800418
Contact: julia.siminskastanny@ulb.be, amin.shavandi@ulb.be
Embedded printing of branched vascular channels – FRESH approach
The lack of a functioning vasculature system within a tissue model can remain a significant barrier to the practical application of such constructs, especially in vivo. Therefore, the project is guided by a hypothesis that the vascularization of the artificial tissue can be overcome by developing new 3D-printing strategies using methacrylated gelatin (GelMA) and hyaluronic acid (HA) biomaterial inks. Conventional extrusion bioprinting requires a high-viscosity bioink to improve the printability and high stiffness to support itself to keep shape fidelity, which subsequently has negative effects on cell viability, migration, or functioning. Nevertheless, low-viscosity inks cannot be printed as standalone structures. In cases as such a support bath that offers temporary and omnidirectional support can stabilize the soft and overhanging material, preventing structure collapse before solidification. As such a new method called freeform reversible embedding of suspended hydrogels (FRESH) can be an alternative way to create vascular channels.
In this project, a support bath consisting of a gelatin slurry will be used to support the creation of vascular-branched scaffolds from gelatin-hyaluronic acid hydrogels. Light curable biomaterial ink will be used to 3D print vessels, by the extrusion of the material in a vertical manner. Following the photocuring, the gelatin thermo-reversible bath will be dissolved, revealing the printed vessel structure. In this project you will focus on the optimization of a crosslinking method employing visible light photo-crosslinking and investigation of inks and hydrogels’ rheological behavior, to determine the most promising formulation for FRESH printing. The last step will cover the fabrication of 3D hydrogel scaffolds (in the form of tubes) and their characterization. Quantitative characterization of embedded printing will be performed using cross-sectional images of the printed structure before and after the removal of the suspension bath. The resulting hydrogels may become promising materials to obtain vessel-like structures with branched organization, which is not possible using conventional 3D printing techniques.
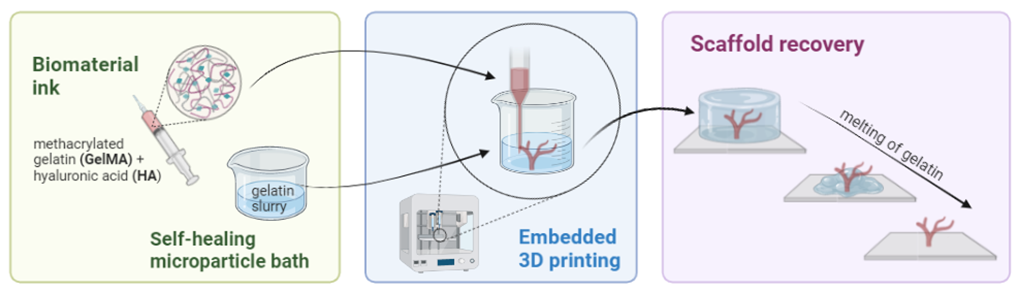
Related literature:
Contact: julia.siminskastanny@ulb.be, amin.shavandi@ulb.be
Contact:pejman.ghaffari.bohlouli@ulb.be , amin.shavandi@ulb.be
Antimicrobial polycaprolactone 2D wound dressing by melt electrowetting method
Most of the available wound dressings are ineffective and suffer from limitations such as poor antimicrobial activity, inability to provide suitable moisture to the wound, and poor mechanical performance. Inappropriate wound dressings can result in a delayed wound healing process. Nano-size range scaffolds have triggered great attention because of their high capability to deliver bioactive agents, high surface area, improved mechanical properties, mimic the extracellular matrix (ECM), and high porosity. Polycaprolactone (PCL), a bioresorbable and biocompatible, synthetic polymer with Food and Drug Administration approval for use in the human body, has been selected as a scaffold material due to its mechanical stability, flexibility, and superior melt processing properties. To increase PCL’s biological functionality bioactive and expand their application, this project aims to conjugate antimicrobial agent on the PCL surface. We hypothesize that by aminolysing of ester groups of PCL, it would replace primary amino groups with guanidine groups that are potent antibacterial agents. To achieve this goal, it is required A) To develop a 2D scaffold by electro writing method based on PCL, B) To aminolyze the surface of the scaffolds by immerging it in isopropyl alcohol solutions of ETDA, EDEA, and HMD (10 wt/vol%) under stirring to ensure that the whole scaffold will be aminolyzed, C) To replace primary amino groups on PCL surface with guanidine groups, D) To study physicochemical properties of antimicrobial PCL scaffolds.
See the following articles for further details about the topic. 1-3
1. Toledo, A.; Ramalho, B.; Picciani, P.; Baptista, L.; Martinez, A.; Dias, M., Effect of three different amines on the surface properties of electrospun polycaprolactone mats. International Journal of Polymeric Materials and Polymeric Biomaterials 2021, 70 (17), 1258-1270.
2. Zhao, Y.-T.; Zhang, J.; Gao, Y.; Liu, X.-F.; Liu, J.-J.; Wang, X.-X.; Xiang, H.-F.; Long, Y.-Z., Self-powered portable melt electrospinning for in situ wound dressing. Journal of nanobiotechnology 2020, 18 (1), 1-10.
3. Piyasin, P.; Yensano, R.; Pinitsoontorn, S., Size-controllable melt-electrospun polycaprolactone (PCL) fibers with a sodium chloride additive. Polymers 2019, 11 (11), 1768.
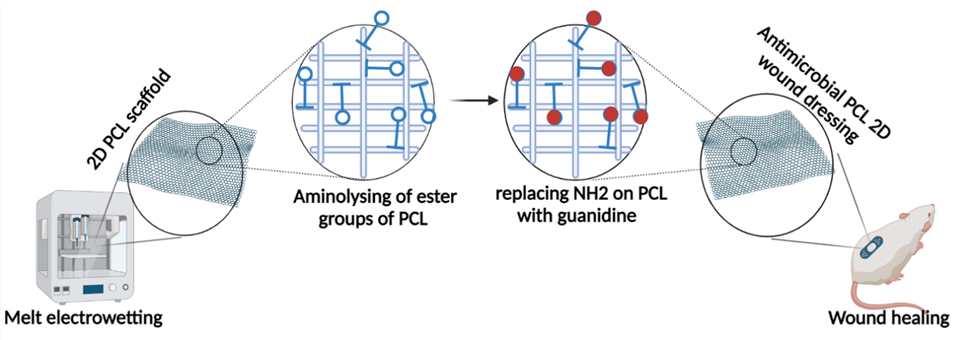
Contact: pejman.ghaffari.bohlouli@ulb.be , amin.shavandi@ulb.be
Antibacterial printable marine-based hydrogels
The design of 3D printable bio-based hydrogels with enhanced mechanical properties and minimal chemical modification can open new opportunities in the field of biomedical applications. A facile and safe approach is proposed to prepare mechanically reinforced chitosan-based hydrogels via a phenolated polyelectrolyte complex (PHEC) and enzyme-mediated crosslinking. PHEC will be formed between phenolated chitosan and alginate, leading to the formation of in situ phenol-functionalized microfibers. By replacing amino groups by phenol groups, the antibacterial activity of chitosan will be decreased, which has a critical role in tissue engineering. Therefore, to compensate the antibacterial activity of the chitosan and increasing antibacterial activity of the system, the guanidine groups will be conjugated on remaining amino groups of chitosan. To achieve this goal, it is required A) To conjugate phenol groups on chitosan and alginate, B) To synthesize a printable hydrogel based on phenolated chitosan and alginate by enzymatic crosslinking, C) To achieve a 3D hydrogel by 3D printing device, D) To conjugate guanidine groups on remaining amino groups of chitosan on 3D hydrogel surface by immersing the gel into guanidine solution, and E) To characterize physicochemical properties of 3D gels.
Please read the following articles for further details about the topic.1-2
1. Jafari, H.; Delporte, C.; Bernaerts, K. V.; Alimoradi, H.; Nie, L.; Podstawczyk, D. A.; Tam, K. C.; Shavandi, A., Synergistically complexation of phenol functionalized polymer induced in-situ microfiber formation for 3D printing of marine-based hydrogel. Green Chemistry 2022.
2. Zhang, X.; Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Lee, M., Supramolecular hydrogels based on nanoclay and guanidine-rich chitosan: injectable and moldable osteoinductive carriers. ACS applied materials & interfaces 2020, 12 (14), 16088-16096.
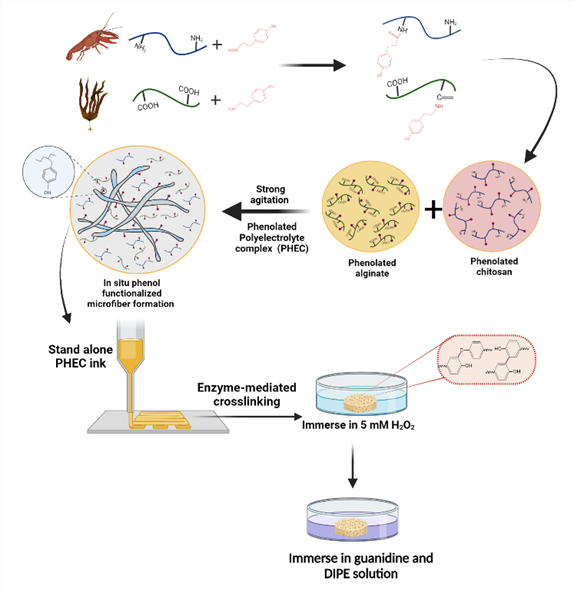
Contact: pejman.ghaffari.bohlouli@ulb.be , amin.shavandi@ulb.be
